What Is A Typical Response Of The Body To Changes In Blood Glucose?
Introduction
Glucose is central to energy consumption. Carbohydrates, lipids, and proteins all ultimately break down into glucose, which and then serves as the primary metabolic fuel of mammals and the universal fuel of the fetus. Information technology serves as the major forerunner for the synthesis of different carbohydrates like glycogen, ribose, and deoxyribose, galactose, glycolipids, glycoproteins, and proteoglycans. On the contrary, in plants, glucose is synthesized from carbon dioxide and h2o (photosynthesis) and stored as starch. At the cellular level, about often, glucose is the final substrate that enters the tissue cells and converts to ATP (adenosine triphosphate).
ATP is the energy currency of the body and is consumed in multiple ways including the active ship of molecules across cell membranes, contraction of muscles and functioning of mechanical work, synthetic reactions that help to create hormones, cell membranes, and other essential molecules, nerve impulse conduction, prison cell partitioning and growth, and other physiologic functions.[i]
Bug of Business organisation
The average fasting blood glucose concentration (no meal within the terminal iii to four hours) is between 80 to 90 mg/dl. On boilerplate, postprandial blood glucose may ascent to 120 to 140 mg/dl, but the body'due south feedback mechanism returns the glucose to normal within 2 hours. During starvation, the liver provides glucose to the torso through gluconeogenesis: synthesizing glucose from lactate and amino acids.
Nosotros tin summarize blood glucose regulation and its clinical significance in the post-obit ways:
-
Liver serving as a buffer for claret glucose concentration
Afterward a repast, there is a rise in blood glucose levels, which raises insulin secretion from the pancreas simultaneously. Insulin causes glucose to deposited in the liver as glycogen; and so, during the adjacent few hours, when claret glucose concentration falls, the liver releases glucose back into the blood, decreasing fluctuations.
Clinical significance: During severe liver disease, it is impossible to maintain blood glucose concentration.
-
Insulin and glucagon work together to maintain normal glucose concentration.
High blood glucose causes insulin secretion, which concomitantly lowers claret glucose levels equally glucose in driven from extracellular to intracellular. Conversely, a autumn in blood glucose stimulates glucagon secretion, which in turn raises blood glucose levels.
Clinical significance: Dumb and bereft insulin secretion leads to diabetes mellitus.
-
CNS control of blood glucose levels
Low claret glucose level is sensed by hypothalamus leading to activation of the sympathetic nervous system to maintain glucose level and avoid astringent hypoglycemia.
-
Event of growth hormone and cortisol.
Prolonged hypoglycemia for hours and days leads to the secretion of growth hormone and cortisol that maintain blood glucose level by increasing fat utilization and decreasing the rate of glucose utilization by cells.[2]
Cellular
Post-obit are the critical steps in the utilization of glucose at the cellular level-
-
Transport of glucose through the jail cell membrane
For glucose to be utilizable in most tissue cells, it needs to be transported across the jail cell membrane into the cytoplasm. Glucose cannot readily diffuse through because of the high molecular weight. Transport is made possible through protein carrier molecules; this is known as facilitated diffusion, and it takes place down the gradient from high concentration to depression concentration.
There is an exception for the gastrointestinal tract and renal tubules. Here, glucose is transported actively by sodium-glucose co-ship against the concentration gradient.
-
Role of insulin in glucose metabolism
The charge per unit of glucose/carbohydrate utilization is under the control of the charge per unit of insulin secretion from the pancreas. Normally, the amount of glucose that can diffuse in the cells is limited except for liver and brain cells. This diffusion is significantly increased by insulin to ten times or more.
-
Phosphorylation of glucose
Every bit soon as glucose enters the jail cell, it becomes phosphorylated to glucose-half dozen-phosphate. This reaction is mediated by glucokinase in the liver and hexokinase in most other cells. This phosphorylating pace serves to capture glucose within the cell. Information technology is irreversible mostly except in liver cells, intestinal epithelial cells, and renal tubular epithelial cells where glucose phosphatase is present in these locations, which is reversible.
This glucose can so either be utilized immediately for the release of free energy through glycolysis, a multi-step procedure to release energy in the form of ATP, or tin can be stored as glycogen(polysaccharide). Liver cells and muscle cells store large amounts of glycogen, for afterward utilization to release glucose by glycogenolysis, i.due east., breakdown of glucose.[3]
Development
In a developing fetus, regulated glucose exposure is imperative to normal growth because glucose is the primary energy grade used by the placenta. In tardily-gestation, fetal glucose metabolism is essential to the development of skeletal muscles, fetal liver, fetal heart, adipose tissue. Three components that are crucial to fetal glucose metabolism are maternal serum glucose concentration, maternal glucose ship to the placenta, which is impacted by the amount of glucose the fetus uses, and finally, fetal pancreas insulin production.
Fetal insulin secretion gradually increases during the gestational period. Pulsatile peaks in glucose level are beneficial to insulin secretion; however, constant hyperglycemia down-regulates insulin sensitivity and glucose tolerance.[4] In mothers with elevated serum glucose levels, adverse fetal impacts include congenital abnormalities, fetal macrosomia, and stillbirth.[5]
Organ Systems Involved
-
Nervous System: The pancreas performs autonomic function through the sympathetic and parasympathetic innervation of the pancreas. The brain itself also houses insulin receptors in multiple regions, including the hypothalamus, cerebellum, hippocampus, among other areas.
-
Pancreas: The pancreas is in the right upper quadrant of the abdomen, behind the stomach. The endocrine functionality of the pancreas regulates glucose homeostasis.
-
Liver: Glycogenesis and gluconeogenesis are the storing and releasing of glucose, respectively. These processes occur using insulin, glucagon, and hepatocyte derived factors.
-
Gut: Hormones in the gut are released in response to the ingestion of nutrients. These hormones are involved in appetite, glucose production, gastric emptying, and glucose removal.
-
Adipocytes: Adipose tissue secretes adipokines, which regulate insulin release through their involvement in glucose metabolism, control of food intake, and insulin factor expression.[half-dozen]
Function
Glucose metabolism involves multiple processes, including glycolysis, gluconeogenesis, and glycogenolysis, and glycogenesis. Glycolysis in the liver is a procedure that involves various enzymes that encourage glucose catabolism in cells. I enzyme, in detail, glucokinase, allows the liver to sense serum glucose levels and to utilise glucose when serum glucose levels ascent, for case, after eating. During periods of fasting, when there is no glucose consumption, for instance, overnight while asleep, the procedure of gluconeogenesis takes place.
Gluconeogenesis happens when in that location is glucose synthesis from non-carbohydrate components in the mitochondria of liver cells. Additionally, during fasting periods, the pancreas secretes glucagon, which begins the glycogenolysis process. In glycogenolysis, glycogen, the stored form of glucose, is released equally glucose. The process of synthesizing glycogen is termed glycogenesis and occurs when excess carbohydrates exist in the liver.[7]
Glucose tolerance is regulated with the circadian cycle. In the morning, humans typically accept their peak glucose tolerance for metabolism. Afternoon and evenings are a trough for oral glucose tolerance. This trough likely occurs because pancreatic beta-cells are also most responsive in the morning—similarly, glycogen storage components peak in the evening. Adipose tissue is virtually sensitive to insulin in the afternoon. The varied timings of fuel utilization throughout the day compose the cycle of glucose metabolism.[eight]
Mechanism
Glycolysis is the most crucial process in releasing free energy from glucose, the end products of which are 2 molecules of pyruvic acrid. It occurs in 10 successive chemical reactions, leading to a net gain of two ATP molecules from ane molecule of glucose.
The overall efficiency for ATP germination is only approximately 40-three per centum, with the remaining 57 percent is lost in the form of heat. The next step is the conversion of pyruvic acid to acetyl coenzyme A, this reaction utilizes coenzyme A, releasing two molecules of carbon dioxide and four hydrogen atoms. No ATP forms at this stage, simply the four released hydrogen atoms participate in oxidative phosphorylation, later releasing six molecules of ATP. The next pace is the breakdown of acetyl coenzyme A and release of energy in the form of ATP in the Kreb's wheel or the tricarboxylic acid cycle, taking place in the cytoplasm of the mitochondrion.[9]
Related Testing
-
HbA1c. The HbA1C value indicates a two to 3 month average of a patient'southward glycemic control. Since the HbA1C value summarizes long-term glycemic control, it is frequently used to evaluate patients with long-standing hyperglycemia, as seen in patients with diabetes, and to forecast the take a chance of diabetic complications.[ten]
-
Fasting Plasma Glucose. Plasma claret glucose level is measured afterward a period of fasting, typically at least 8 hours. A value greater than 126 mg/dL is associated with diabetes.[11]
-
Random Plasma Glucose. A random plasma glucose measurement is sampled old after dietary intake was last ingested. A value greater than 200 mg/dL is highly suggestive of diabetes.[12]
-
Oral Glucose Tolerance Test. All pregnant women should receive gestational diabetes mellitus (GDM) screening through an orally consumed glucose challenge and subsequent plasma claret glucose measurement.[xiii]
-
C-Peptide. C-peptide is a quantitative measurement of beta-cell function in an individual's pancreas. Measured via urine or serum samples, a C-peptide value aids in the evaluation and management of diabetes.[xiv]
-
Autoantibody. Presence of autoantibodies, including islet autoantibody, insulin autoantibody, insulinoma-associated antigen-ii autoantibodies, anti-glutamic acid decarboxylase (GAD) autoantibodies, amongst others are suggestive of auto-immune response as is seen in type 1 diabetes.[15][xvi]
Pathophysiology
Although not completely understood, Type 1 and Type two diabetes differ in their pathophysiology. Both are considered polygenic diseases, meaning multiple genes are involved, likely with multifactorial ecology influences including gut microbiome composition and environmental pollutants, amid others. Type i diabetes is the autoimmune destruction of the trunk's pancreatic beta cells, which produce insulin hormone. Without the insulin hormone, the body is unable to regulate claret glucose control. Type 1 diabetes more normally presents in childhood and persists through adulthood, equally affects males and females, and has the highest prevalence of diagnosis in European White race individuals. Life expectancy for an individual with Blazon 1 diabetes is reduced by an estimated thirteen years.
Type 2 diabetes results when pancreatic beta cells cannot produce plenty insulin to meet metabolic needs. Additionally, equally adipose cells are deposited in the patient'south liver and musculus, insulin resistance becomes a prominent feature of Type 2 diabetes. Therefore, individuals with more than adipose degradation, typically with college body fat content and an obese BMI, more unremarkably accept type 2 diabetes.
Type 2 diabetes is more mutual, every bit 95% of individuals with overall diabetes have Type ii Diabetes. Blazon 2 diabetes is more common amongst adult and older developed populations; notwithstanding, youth are demonstrating rise rates of type 2 diabetes. Blazon 2 diabetes is slightly more than common in males (6.ix%) than in females (5.nine%). It is also more common in individuals of Native American, African American, Hispanic, Asian, and Pacific Islander race or ethnicity.[17]
Clinical Significance
Poor glucose metabolism leads to diabetes mellitus. According to the American Diabetes Association, the prevalence of diabetes in the yr 2015 was ix.4%, with people crumbling 65 and older forming the largest grouping. Every twelvemonth, 1.v meg Americans receive a diagnosis of diabetes. Equally the seventh-highest cause of mortality in the U.s.a., diabetes mellitus poses a apropos healthcare claiming with large amounts of yearly expenditures, morbidity, and decease.
Diabetes classifies into ii types-
Blazon 1 DM- due to deficient insulin secretion. Key features of this type are-
-
Immune-mediated in over xc% cases.
-
It can occur in whatever historic period group, only it is about mutual in children and young adults.
-
Circulating insulin is nigh absent, leading to a catabolic state with exogenous insulin required for treatment.
Blazon two DM-due to insulin resistance with a defect in compensatory insulin secretion. Cardinal features of this type are-
-
This condition occurs predominantly in adults just now too increasingly presents in children and adolescents.
-
Genetic and environmental factors combine, leading to both insulin resistance and beta-cell loss.
-
Treatment entails lifestyle changes and oral anti-diabetic drugs.
Uncontrolled diabetes poses a significantly increased risk of developing macrovascular disease, especially coronary, cerebrovascular, and peripheral vascular disease. Information technology besides increases the chances of microvascular illness, including retinopathy, nephropathy, and neuropathy.[18]
Review Questions
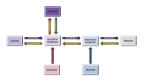
Figure
Diagram of the human relationship between the processes of saccharide metabolism, including glycolysis, gluconeogenesis, glycogenesis, glycogenolysis, fructose metabolism, and galactose metabolism. Contributed by Wikimedia User: Eschopp, CC By-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0, (more than...)
References
- ane.
-
Jaiswal N, Gavin MG, Quinn WJ, Luongo TS, Gelfer RG, Baur JA, Titchenell PM. The role of skeletal musculus Akt in the regulation of musculus mass and glucose homeostasis. Mol Metab. 2019 Oct;28:1-13. [PMC free article: PMC6822261] [PubMed: 31444134]
- 2.
-
Chen Y, Zhao X, Wu H. Metabolic Stress and Cardiovascular Illness in Diabetes Mellitus: The Role of Poly peptide O-GlcNAc Modification. Arterioscler Thromb Vasc Biol. 2019 Oct;39(10):1911-1924. [PMC free commodity: PMC6761006] [PubMed: 31462094]
- three.
-
Taneera J, Dhaiban Southward, Mohammed AK, Mukhopadhyay D, Aljaibeji H, Sulaiman N, Fadista J, Salehi A. GNAS gene is an important regulator of insulin secretory capacity in pancreatic β-cells. Gene. 2019 Oct 05;715:144028. [PubMed: 31374326]
- 4.
-
Hay WW. Placental-fetal glucose commutation and fetal glucose metabolism. Trans Am Clin Climatol Assoc. 2006;117:321-39; discussion 339-40. [PMC gratis article: PMC1500912] [PubMed: 18528484]
- 5.
-
Schaefer-Graf U, Napoli A, Nolan CJ., Diabetic Pregnancy Study Group. Diabetes in pregnancy: a new decade of challenges ahead. Diabetologia. 2018 May;61(5):1012-1021. [PMC complimentary article: PMC6448995] [PubMed: 29356835]
- 6.
-
Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016 Mar 11;48:e219. [PMC free article: PMC4892884] [PubMed: 26964835]
- 7.
-
Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016 Mar 11;48:e218. [PMC free commodity: PMC4892876] [PubMed: 26964834]
- viii.
-
Poggiogalle E, Jamshed H, Peterson CM. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism. 2018 Jul;84:11-27. [PMC free commodity: PMC5995632] [PubMed: 29195759]
- 9.
-
Tozzi Thousand, Hansen JB, Novak I. Pannexin-i mediated ATP release in adipocytes is sensitive to glucose and insulin and modulates lipolysis and macrophage migration. Acta Physiol (Oxf). 2020 Feb;228(ii):e13360. [PubMed: 31400255]
- ten.
-
Schnell O, Crocker JB, Weng J. Bear on of HbA1c Testing at Point of Care on Diabetes Management. J Diabetes Sci Technol. 2017 May;eleven(3):611-617. [PMC complimentary commodity: PMC5505423] [PubMed: 27898388]
- 11.
-
Eun YM, Kang SG, Song SW. Fasting plasma glucose levels and coronary artery calcification in subjects with impaired fasting glucose. Ann Saudi Med. 2016 Sep-Oct;36(5):334-340. [PMC costless article: PMC6074322] [PubMed: 27710985]
- 12.
-
Barasch A, Gilbert GH, Spurlock N, Funkhouser E, Persson LL, Safford MM., DPBRN Collaborative Group. Random plasma glucose values measured in community dental practices: findings from the dental practice-based research network. Tex Paring J. 2013 Apr;130(4):291-seven. [PubMed: 23767158]
- thirteen.
-
Garrison A. Screening, diagnosis, and management of gestational diabetes mellitus. Am Fam Doc. 2015 Apr 01;91(vii):460-7. [PubMed: 25884746]
- xiv.
-
Leighton Due east, Sainsbury CA, Jones GC. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther. 2017 Jun;eight(3):475-487. [PMC free article: PMC5446389] [PubMed: 28484968]
- 15.
-
Regnell SE, Lernmark Å. Early prediction of autoimmune (type 1) diabetes. Diabetologia. 2017 Aug;60(8):1370-1381. [PMC free article: PMC5491594] [PubMed: 28550517]
- sixteen.
-
Pippitt K, Li M, Gurgle HE. Diabetes Mellitus: Screening and Diagnosis. Am Fam Physician. 2016 January 15;93(2):103-9. [PubMed: 26926406]
- 17.
-
Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop Fifty, Groop PH, Handelsman Y, Insel RA, Mathieu C, McElvaine AT, Palmer JP, Pugliese A, Schatz DA, Sosenko JM, Wilding JP, Ratner RE. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes. 2017 Feb;66(ii):241-255. [PMC costless article: PMC5384660] [PubMed: 27980006]
- 18.
-
Zhang N, Jiang H, Bai Y, Lu 10, Feng M, Guo Y, Zhang S, Luo Q, Wu H, Wang Fifty. The molecular mechanism report of insulin on proliferation and differentiation of osteoblasts under loftier glucose conditions. Cell Biochem Funct. 2019 Jul;37(5):385-394. [PubMed: 31140646]
Source: https://www.ncbi.nlm.nih.gov/books/NBK560599/
Posted by: sanchezalmle1941.blogspot.com


0 Response to "What Is A Typical Response Of The Body To Changes In Blood Glucose?"
Post a Comment